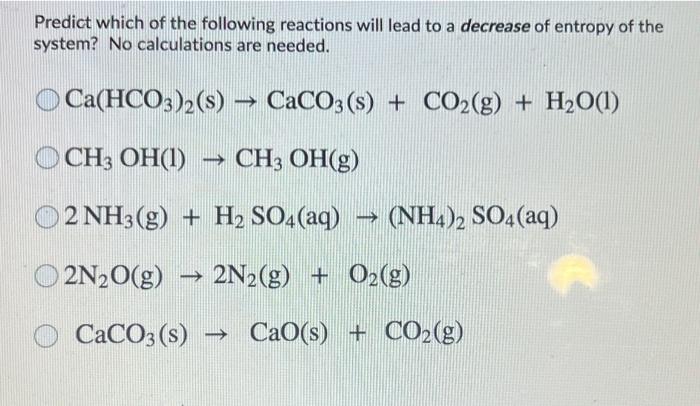
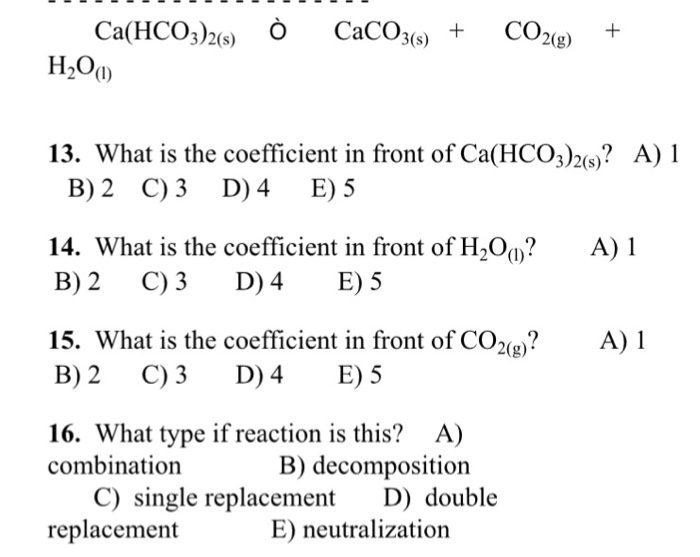
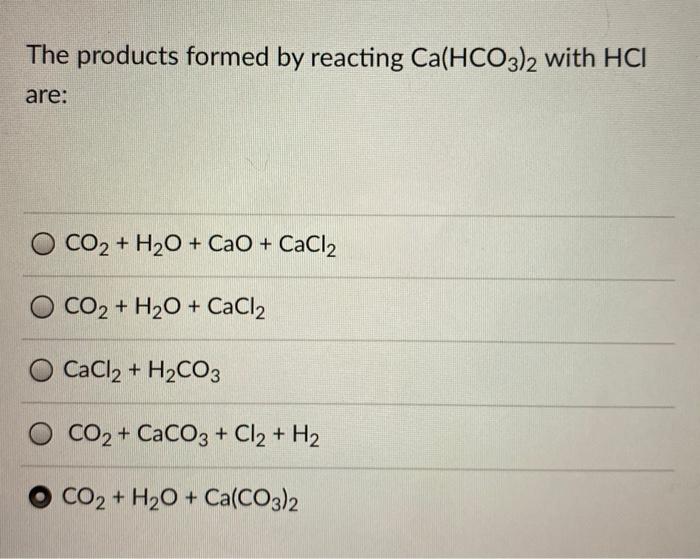
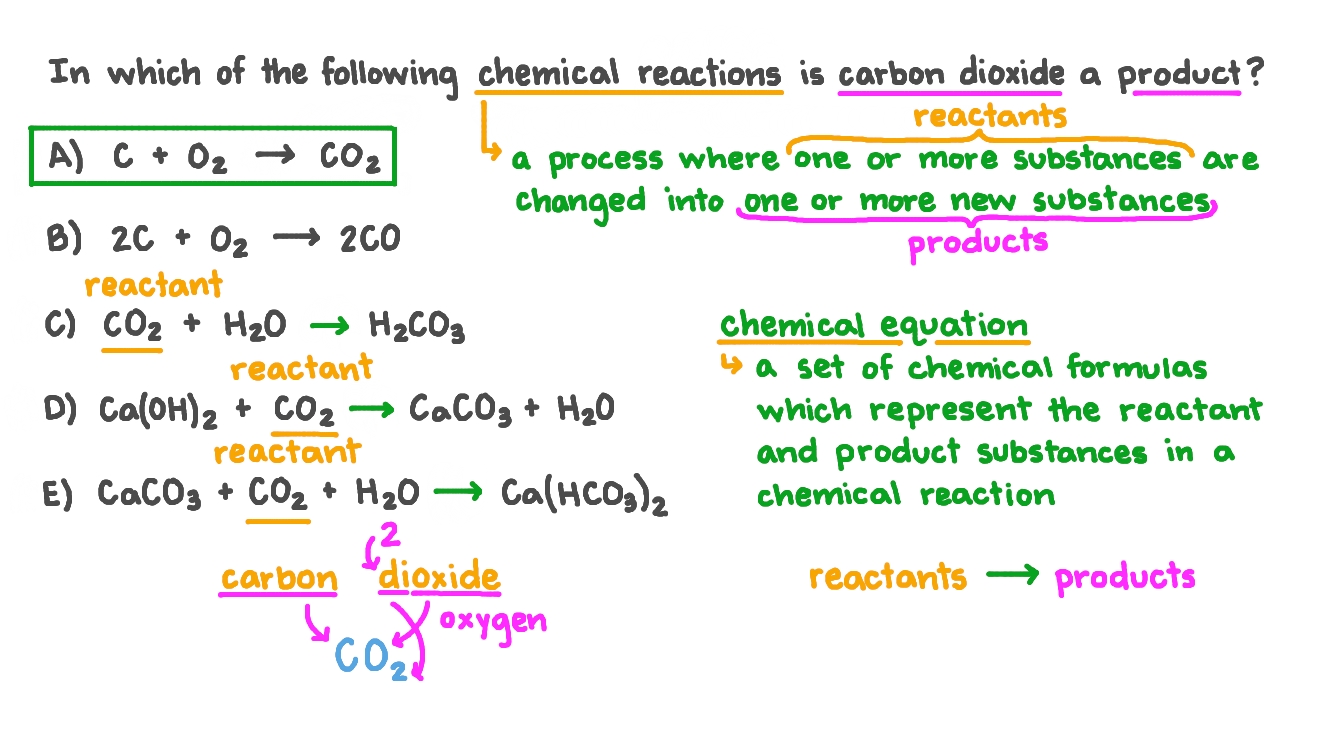
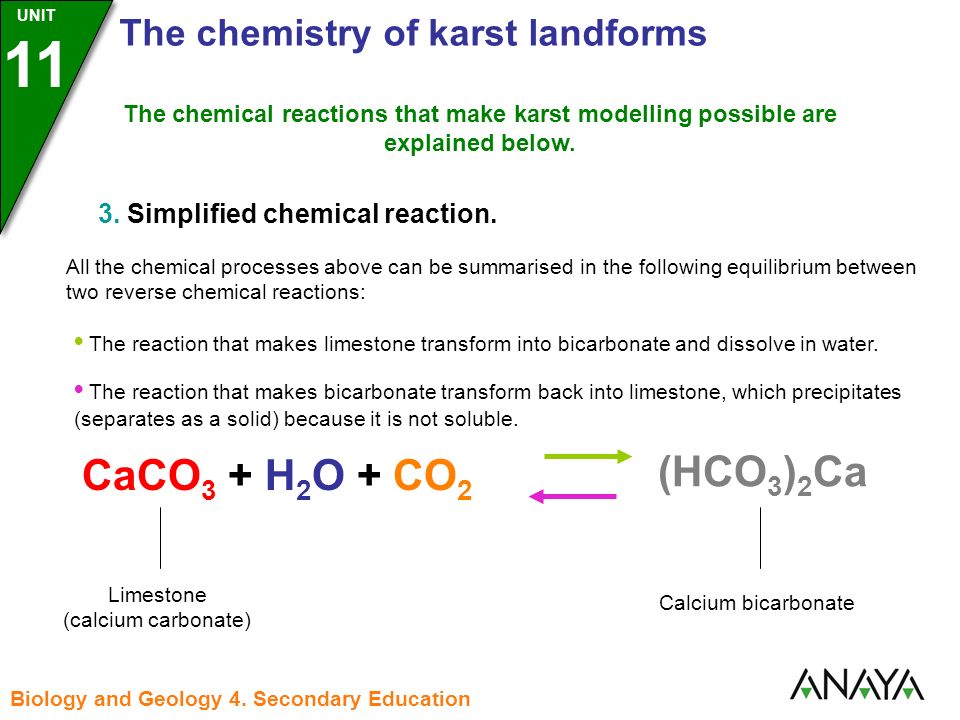
Ca(HCO3)2 decomposes as Ca(HCO3)2(s)→CaCO3 (s)+H2O (g)+CO2 (g). Total pressure at equilibrium is found to be 0.12 bar. Thus, Kp is ?

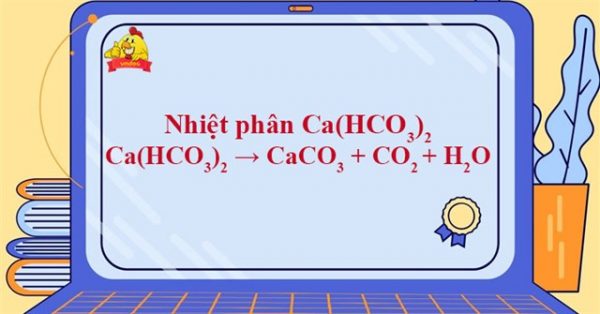
How to balance (HCO3)2=CaCO3+CO2+H2O|Chemical equation Ca(HCO3)2=CaCO3+CO2+ H2O|Ca(HCO3)2= - YouTube

SOLVED: .From the following chemical reaction which reaction will occur when excess of CO2 is passed through lime water? (a) Ca(OH)2 + CO2 CaCO3 + H2O (b) CaCO3 + H2O + CO2

☆ Balance the chemical Reaction -1)CaCo3 + H₂O + CO2------- Ca(HCO3)22) NaOH + HCl ------ NaCl + H₂O - Brainly.in

PDF) Novel Method of Generation of Ca(HCO3)2 and CaCO3 Aerosols and First Determination of Hygroscopic and Cloud Condensation Nuclei Activation Properties

How to Balance Ca(HCO3)2 = CaCO3 + CO2 + H2O (Decomposition of Calcium hydrogen carbonate) - YouTube

SOLVED: The formation of hydrogen sulfide at 300C Ca(HCO3)2 (s) v HzO (g) + COz (g) + CaCO3 (s) Kp = 2.88 If a 82 gram sample of Ca(HCOz is put into